Resources
Below are references mentioned during the roundtable discussion. These items include scientific articles, abbreviations, news articles, panelists affiliations and graphical representations of chemical structures.
KS Insights: Digital Health: From Science to Application (A5)
About Keystone Symposia Insights
Keystone Symposia Insights are panel discussions recorded on digital video that feature Keystone Symposia meeting organizers discussing the state of their respective fields. These thought leaders provide perspectives on current and future trends.
The panel is asked to discuss the following topics:
- What is the state of this field? (What did we learn at the meeting?)
- Where is the field heading?
- What are the opportunities and challenges ahead?
Articles
#WeHeartHackers
A new FDA-led initiative with the Biohacking Village at DEF CON that encourages collaboration between white hat security researchers and med device manufacturers
The Doctor Prescribes Video Games and Virtual Reality Rehab
Andy Coravos, November 20, 2018
A WIRED op-ed on software and AI that measure, diagnose and treat
https://www.wired.com/story/prescription-video-games-and-vr-rehab/
Peer review could help smoke out the next Theranos
Eli Cahan, Ioana A. Cristea, and John P.A. Ioannidis, January 29, 2019
https://www.statnews.com/2019/01/29/peer-review-health-care-unicorns-theranos/
BioHackers Assemble! FDA and Medical Device Manufacturers say #WeHeartHackers at DEFCON 27
Lisa Suennen, April 14, 2019
The Elements of Informed Consent Toolkit
Sage Bionetworks
http://sagebionetworks.org/in-the-news/elements-informed-consent/
The Elements of Informed Consent is a toolkit that will help researchers think through what information participants should receive as part of the consenting process in order to make an informed decision about whether or not to join a study. Developed by the Governance Team at Sage Bionetworks, the toolkit shares basic information and best practices for developing an effective consenting process. We believe that informed participants make the best participants, because they understand the study, its risks and benefits, and how their data will be treated.
Program: Mobile Clinical Trials (MCT)
John Hubbard, et al., Clinical Trials Transformation Initiative (CTTI)
https://www.ctti-clinicaltrials.org/projects/mobile-technologies
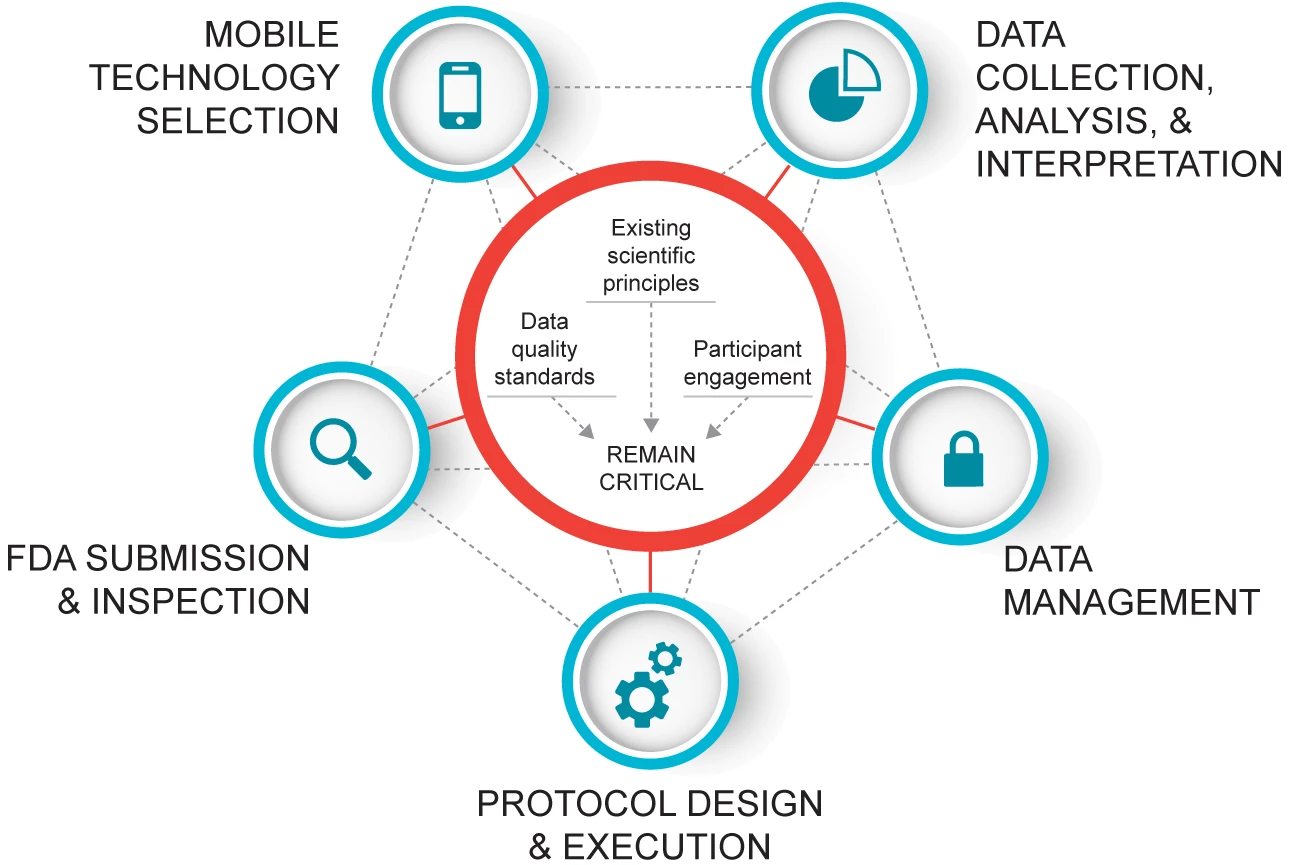
CTTI Recommendations: Developing Novel Endpoints Generated by Mobile Technology for Use in Clinical Trials
Clinical Trials Transformation Initiative (CTTI)
https://www.ctti-clinicaltrials.org/files/novelendpoints-recs.pdf
Use Case for Developing Novel Endpoints Generated Using Mobile Technology: Duchenne Muscular Dystrophy
Clinical Trials Transformation Initiative (CTTI)
https://www.ctti-clinicaltrials.org/files/usecase-duchenne.pdf